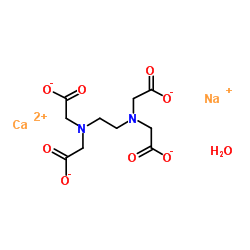
Disodium Edta Structure

Ethylenediaminetetraacetic Acid Wikipedia

Edta Openwetware
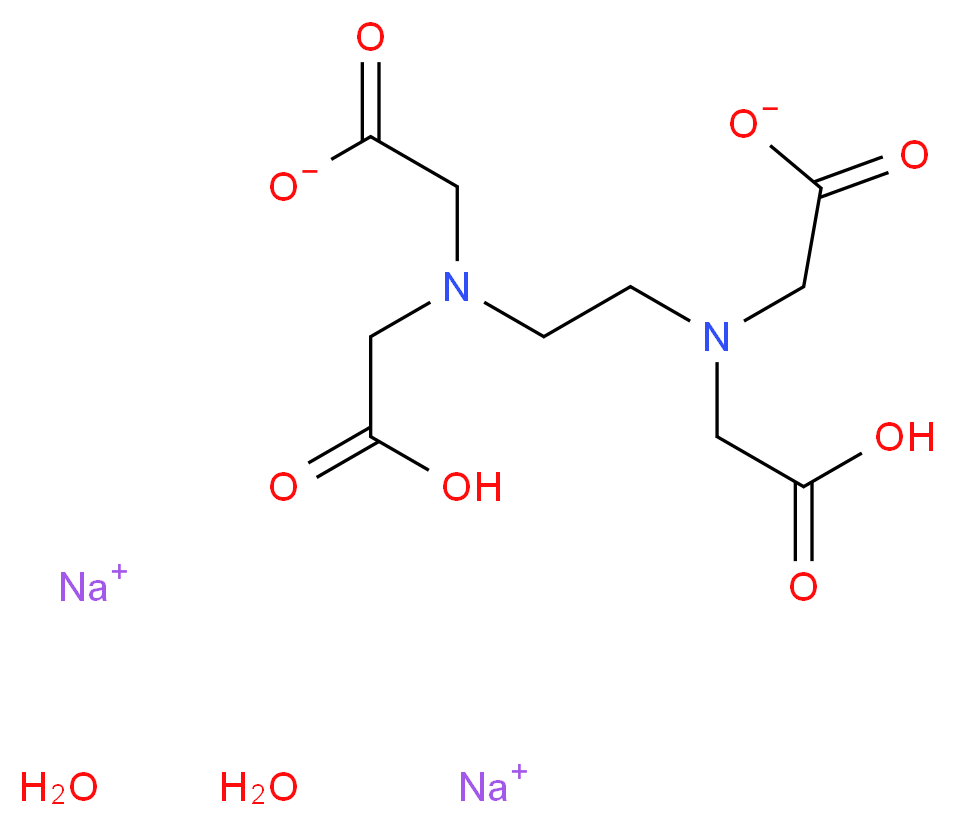
6381 92 6 Ethylenediaminetetraacetic Acid Disodium Salt Dihydrate Alfa Aesar Edta Di

Structures Of Edta Disodium Salt Left And Metal Edta Complexes Right Download Scientific Diagram

Ethylenediaminetetraacetic Acid Disodium Nickel Ii Salt 55 1 Tokyo Chemical Industry Co Ltd Apac
Disodium Edta Dihydrate C10h18n2na2o10 Chemspider
EDTA was first used in the 1950s for the treatment of heavy metal poisoning Calcium disodium EDTA chelation removes heavy metals and minerals from the blood, such as lead, iron, copper, and calcium, and is approved by the FDA for use in treating lead poisoning and toxicity from other heavy metals.

Disodium edta structure. 005–011–01–1 and 005–011–02–9), CLH information cannot be displayed in the InfoCard as the difference between the CLH classifications requires manual interpretation or. Structure, properties, spectra, suppliers and links for DISODIUM EDTA DIHYDRATE,. Ethylenediaminetetraacetic Acid Disodium Salt, N,N'1,2ethanediylbis(N(carboxymethyl)glycine) edetic acid Disodium Salt, Disodium Edetate, Metaclaw, Trilon B, Versene 2, Dissolvine, Titriplex, etc Molecular Formula C 10 H 14 O 8 N 2 Na 22H 2 O Molecular Structure Molecular Weight 3722 Appearance White Crystalline Powder Solubility.
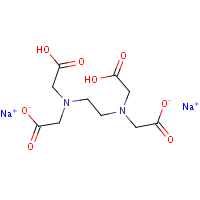
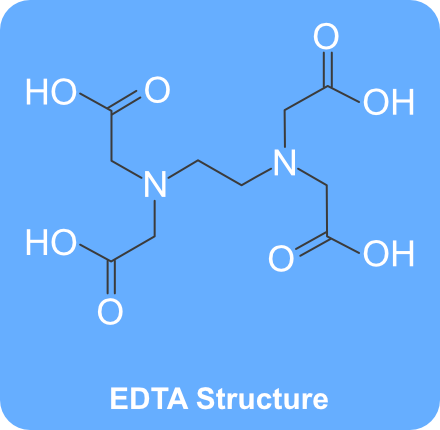
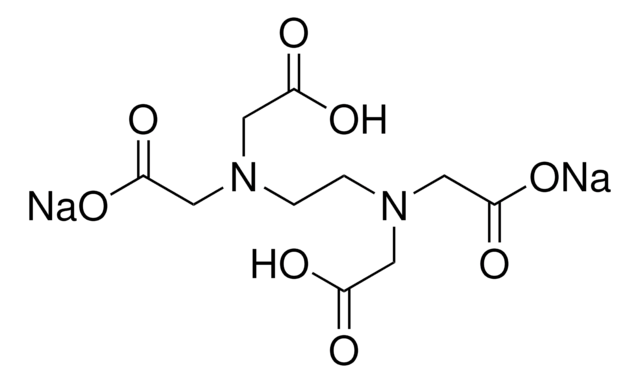
Disodium EDTA is a form of EDTA with two sodium cations It is a heavy metal chelating agent and present as a dry powder The general structure of EDTA contains four negatively charged oxygen atoms Out of the four, two oxygen atoms of EDTA remain combined with two sodium cations to form disodium EDTA. EDTA, Disodium Salt, Dihydrate, Molecular Biology Grade CAS Calbiochem 1 Product Result. Disodium EDTA C10H16N2Na2O8 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Edetate disodium anhydrous Commonly known or available as Edetate disodium Accession Number DB Description Edetate disodium anhydrous is a polyvalent chelating agent used to treat hypercalcemia and digitalis toxicity associated ventricular arrhythmias 1 Type Small Molecule Groups Approved, Vet approved Structure. EDTA is a dry, white crystalline powder EDTA is a hexadentate ligand, which means that it creates 6 bonds with a central metal ionWhen it bonds with a calcium ion, it becomes EDTA calcium disodium EDTA calcium disodium can then chelate other metal ions by exchanging its calcium ion for another metal ion that has a greater affinity for the EDTA molecule. Synonym (Ethylenedinitrilo)tetraacetic acid disodium salt, EDTA disodium salt, EDTANa 2, Ethylenediaminetetraacetic acid disodium salt solution Linear Formula CH 2 N(CH 2 CO 2 Na)CH 2 CO 2 H 2.
Disodium EDTA dihydrate is common used form in biology laboratories EDTA structure Applications A variant of EDTA (Sodium Calcium EDTA) is used to treat lead (Pb) and mercury (Hg) poisoning Used in dental procedures to remove inorganic depositions and calcification inside the root canal. DISODIUM EDTA DIHYDRATE Molecular Formula C 10 H 18 N 2 Na 2 O 10;. SRRLDCNCJFKVFJUHFFFAOYSAJ Disodium cobalt EDTA Similar structures search, synonyms, formulas, resource links, and other chemical information.
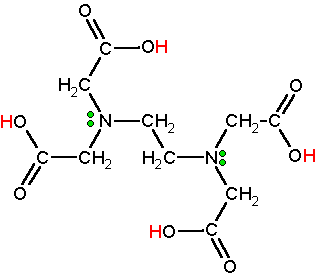
Edetate Disodium is the disodium salt form of edetate, a heavy metal chelating agent with antihypercalcemic and antiarrhythmic properties Edetate, a heavy metal antagonist, chelates divalent and trivalent metals, forming soluble stable complexes which are readily excreted by the kidneys, thereby can be used to lower serum calcium concentrations In addition, this agent exerts a negative. Calcium disodium EDTA is approved by the FDA for use in lead poisoning and has been the mainstay of treatment for childhood lead poisoning since the 1950s 4 The second drug, disodium EDTA, is approved for use in patients with rhythm disorders from drug intoxication such as digitalis where there is hypercalcemia Calcium disodium EDTA. Ethylenediaminetetraacetic acid is an aminopolycarboxylic acid with the formula CH2N(CH2CO2H)22 This white, watersoluble solid is widely used to bind to iron and calcium ions It binds these ions as a hexadentate chelating agent EDTA is produced as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA Ethylenediaminetetraacetic acid Names Systematic IUPAC name 2,2′,2″,2‴tetraacetic acid Other names EthyleneDiamineTetraAcetic acid N,N′Ethane1,2.
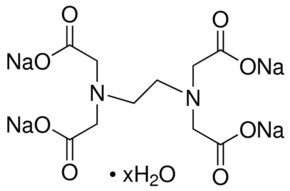
EDTA, Ethylenediaminetetraacetic acid is a molecule which complexes metal ions in aqueous environments It is available in four neutralizations, two of which, Disodium EDTA and Tetrasodium EDTA, are commonly used in cosmetics Generally, the choice of which product to use is determined by the intended pH of your product. EDTA, Disodium Salt, Dihydrate, Molecular Biology Grade CAS Calbiochem 1 Product Result. EDTA is commercially available in several forms Here are few EDTA (Molecular Weight ) EDTNa4H₂O (Molecular Weight ) EDTNa2H ₂ O ( Molecular Weight ) EDTK2H₂O (Molecular Weight) EDTNaCa EDTNaMg Disodium EDTA dihydrate is common used form in biology laboratories.
Molecular structure The molecular structure is based on structures generated from information available in ECHA’s databases EDTA disodium salt, EDTANa2, Edathamil, Edetate disodium salt, Sequestrene Na2, Ethylenediaminetetraacetic acid disodium salt Registration dossier EDTA 2Na. Disodium magnesium edta Molecular Formula C 10 H 12 MgN 2 Na 2 O 8;. Calcium disodium EDTA is a popular food additive and chelating agent Even though it's considered safe, it may cause side effects when consumed in high doses.
Formula C 10 H 14 N 2 O 8 Na 2 • 2H 2 O Synonyms Dihydrate, Na 2 EDTA Hydration Dihydrate Shipping and Storage Storage Temp. NaLn(EDTA)(H 2 O) 35H 2 O (Ln = La, Nd, Eu) are isostructural, with ninecoordinate lanthanides 642 KYb(EDTA)(OH 2) 25H 2 O contains eightcoordinate ytterbium 643 Structures of several MLn(EDTA)(H 2 O) n (M = alkali metal;. EDTA disodium salt (anhydrous) ( CHEBI ) is a organic sodium salt ( CHEBI ) Incoming EDTA disodium salt dihydrate ( CHEBI ) has part EDTA disodium salt (anhydrous) ( CHEBI) IUPAC Name disodium 2,2',2'',2''' (ethane1,2diyldiammonio)tetraacetate Synonyms Sources.
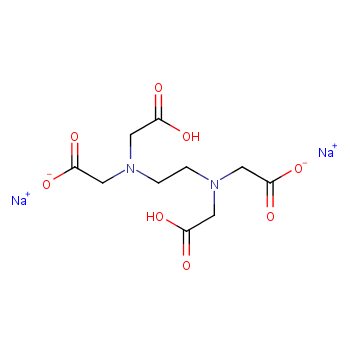
Disodium EDTA is a form of EDTA composed of two sodium cations EDTA generally has four negatively charged oxygen atoms in its structure In disodium EDTA, two of these oxygen atoms are combined with two sodium cations Disodium EDTA is a byproduct of the synthesis of EDTA. Structure General Activity Publications Names 9 Identifiers 4 Related Substances 3 DISODIUM EDTACOPPER 6V475AX06U DISODIUM EDTACOPPER INCI Sources Common Name English NSC7346 Sources Code English BOVICU Sources Brand Name English. EDTA disodium salt C10H14N2Na2O8 CID 1300 structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Ethylenediaminetetraacetic Acid Disodium Salt, N,N'1,2ethanediylbis(N(carboxymethyl)glycine) edetic acid Disodium Salt, Disodium Edetate, Metaclaw, Trilon B, Versene 2, Dissolvine, Titriplex, etc Molecular Formula C 10 H 14 O 8 N 2 Na 22H 2 O Molecular Structure Molecular Weight 3722 Appearance White Crystalline Powder Solubility. These two ingredients have very similar uses in skincare but have some small differences in the way they are used The main two differences between the two ingredients are that they have a different number of sodium cations;. EDTA Structure (Source – PubChem) What is EDTA?.
The Chemistry of Disodium EDTA Disodium EDTA has the chemical formula of CH2N (CH2CO2H)22, the molecular formula of C10H16N2O8, and is a major chelating agent from which all of its applications. 005–011–01–1 and 005–011–02–9), CLH information cannot be displayed in the InfoCard as the difference between the CLH classifications requires manual interpretation or. If the substance is covered by more than one CLH entry (eg disodium tetraborate EC no 215–540–4, is covered by three harmonisations:.
EDTA helps to improve the foaming of cleansers, soaps, and body washes Two primary forms of EDTA are frequently used in personal care products, Tetrasodium EDTA and Disodium EDTA The main difference between tetrasodium EDTA and disodium EDTA is the structure of the molecules and the pH. Tetrasodium EDTA has four sodium cations, and disodium EDTA has two. Tetrasodium EDTA has four sodium cations, and disodium EDTA has two.
EDTA (short for Ethylenediaminetetraacetic Acid) is a metal binding compound used in skin care formulations to prevent deterioration EDTA (ethylenediaminetetraacetic acid) od often used in one of its many salt forms such as Calcium Disodium EDTA, Diammonium EDTA, Dipotassium EDTA,. Substance name EDTA, Disodium, Dihydrate CASNo Product code LC Formula C10H14N2O8Na2·2H2O Synonyms Ethylenediaminetetraacetic acid, disodium salt, dihydrate 12 Recommended use and restrictions on use Use of the substance/mixture For laboratory and manufacturing use only. These two ingredients have very similar uses in skincare but have some small differences in the way they are used The main two differences between the two ingredients are that they have a different number of sodium cations;.
EDTA, Ethylenediaminetetraacetic acid is a molecule which complexes metal ions in aqueous environments It is available in four neutralizations, two of which, Disodium EDTA and Tetrasodium EDTA, are commonly used in cosmetics Generally, the choice of which product to use is determined by the intended pH of your product. Average mass Da;. In EDTA the 4 hydrogen atoms of the carboxylic acid group are in a form of acidic hydrogens and can be replaced by metals like sodium or calcium to form salts like, disodium EDTA, calcium disodium EDTA or tetrasodium salt of Ethylene diamine tetraacetic acid (exists in form of a hydrate).
EDTA, Ethylenediaminetetraacetic acid is a molecule which complexes metal ions in aqueous environments It is available in four neutralizations, two of which, Disodium EDTA and Tetrasodium EDTA, are commonly used in cosmetics Generally, the choice of which product to use is determined by the intended pH of your product. Structure Find Similar Structures Molecular Formula C10H12O8CaN2Na2·2H2O or C10H14CaN2Na2O Synonyms Calcium disodium EDTA CalciumdisodiumEDTA DS. Global Disodium EDTA Market Structure Send Message Key Questions Answered in the Report What is the growth rate of the global disodium EDTA market?.
Magnesium EDTA, however, is not commercially available in either of these standards Even after 68 years of research into EDTA chemistry, the calcium disodium form continues to come out on top in regards to lowest toxicity, longest history of safe use in humans and other animals, and as the safest and most effective oral chelator. Structure of EDTA EDTA is a dry, white crystalline powder EDTA is a hexadentate ligand, which means that it creates 6 bonds with a central metal ion When it bonds with a calcium ion, it becomes EDTA calcium disodium. Ln = lanthanide) show that the coordination number depends upon the ionic radii of both the lanthanide and the alkali metal 644 The complex (guanidinium) 2 Eu(EDTA)F(H 2 O) 22H 2 O contains ninecoordinate europiums linked to two fluorine bridges 645 UV.
Average mass Da;. Empirical Formula (Hill Notation) C 10 H 14 N 2 Na 2 O 8 · 2H 2 O Molecular Weight Beilstein/REAXYS Number NACRES N4 You have selected the maximum number of product attributes (3) to compare. General description Ethylenediaminetetraaceti c acid (EDTA) is an aminopolycarboxylic acid and a hexadentate ligand It chelates with metal ions, especially with cations to form an octahedral complex Ethylenediaminetetraaceti c acid disodium salt (EDTA) is a blood anticoagulant and contributes to the pathogenesis of pseudothrombocytopenia It chelates with calcium in the blood and inhibits.
The global disodium EDTA market is projected to grow at a CAGR of 43% between 21 and 26. Disodium EDTA dihydrate C10H18N2Na2O10 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. The key difference between disodium EDTA and tetrasodium EDTA is that disodium EDTA has a pH lower than 7 while tetrasodium EDTA has a pH greater than 7 EDTA is a chelating agentTherefore, it has the potential to bind with metal ions such as calcium and magnesiumEDTA stands for Ethylenediaminetetraacetic acid It results in sequestration of metal ions.
Molecular structure The molecular structure is based on structures generated from information available in ECHA’s databases EDTA disodium salt, EDTANa2, Edathamil, Edetate disodium salt, Sequestrene Na2, Ethylenediaminetetraacetic acid disodium salt Registration dossier EDTA 2Na. Add to it 100 ml of acetic acidammonium acetate buffer, 2500 ml of alcohol and 2 ml of dithizone solution as an indicator Titrate the excess of disodium edetate with 0025 M zinc sulphate until the resulting solution turns rose pink in colour Each millilitre of 005 M disodium edetate consumed is equivalent to 1045 mg of Bi. Monoisotopic mass Da;.
If the substance is covered by more than one CLH entry (eg disodium tetraborate EC no 215–540–4, is covered by three harmonisations:. Molecular structure The molecular structure is based on structures generated from information available in ECHA’s databases EDTA disodium salt, EDTANa2, Edathamil, Edetate disodium salt, Sequestrene Na2, Ethylenediaminetetraacetic acid disodium salt Registration dossier EDTA 2Na. The disodium form of EDTA is approved by the FDA for this use, but healthcare providers generally prefer other treatments such as lidocaine or phenytoin (Dilantin) These treatments are considered.
Edetate disodium (EDTA) is a chelating (KEElateing) agent A chelating agent is capable of removing a heavy metal, such as lead or mercury, from the blood EDTA is used to lower blood levels of calcium when they have become dangerously high. EDTA is a chelating agent, that is, it incorporates a heavy metal ion into a ring structure The biological halflife of EDTA in humans is about one hour 28 About 12% remains in the body after 24 hours and 05% after 48 hours. The Chemistry of Disodium EDTA Disodium EDTA has the chemical formula of CH2N (CH2CO2H)22, the molecular formula of C10H16N2O8, and is a major chelating agent from which all of its applications.
Monoisotopic mass Da;. Advanced Search Structure Search (Ethylenedinitrilo)tetraacetic acid calcium disodium salt, EDTA calcium disodium salt, Edathamil, Ethylenediaminetetraacetic acid calcium disodium salt Linear Formula C 10 H 12 N 2 O 8 CaNa 2 Molecular Weight CAS Number. Empirical Formula (Hill Notation) C 10 H 14 N 2 Na 2 O 8 · 2H 2 O Molecular Weight Beilstein/REAXYS Number NACRES N4 You have selected the maximum number of product attributes (3) to compare.
CAS Molecular Formula C10H18N2Na2O10 Molecular Weight (g/mol) InChI Key OVBJJZOQPCKUORUHFFFAOYSAL Synonym edta disodium salt,calex decalcifier,buffer solution, ph 1000,sodium di ethylenediamine tetraacetate dihydrate,ethylenediamine tetraacetic acid, disodium salt dihydrate,ethylenediamine tetraacetic acid, disodium salt, standard solution,sodium di. Calcium disodium EDTA is a common food additive and an ingredient in cosmetic and industrial products This article reviews calcium disodium EDTA, its applications, safety and side effects. SRRLDCNCJFKVFJUHFFFAOYSAJ Disodium cobalt EDTA Similar structures search, synonyms, formulas, resource links, and other chemical information.
Edetate Calcium Disodium 99 Hplc Selleck Others Qcfile

Difference Between Disodium Edta And Tetrasodium Edta Compare The Difference Between Similar Terms

Ethylenediaminetetraacetic Acid Wikiwand
Disodium Monocalcium Edta Cas 62 33 9 Chemsrc
Q Tbn And9gcrsot6kcjbizkbc6jfiqa4naqjslopbfhjtobjyoey Usqp Cau

Edta Disodium C10h14n2na2o8 Pubchem
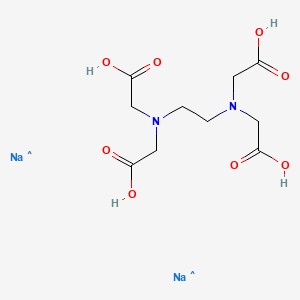
Disodium Edta C10h16n2na2o8 Pubchem
Disodium Edta Hazardous Agents Haz Map

Ca Ii Edta Dojindo

Mg Ii Edta Dojindo
Chemlab Truman Edu Files 15 07 Edta Pdf
Zinc Disodium Edta 21 9

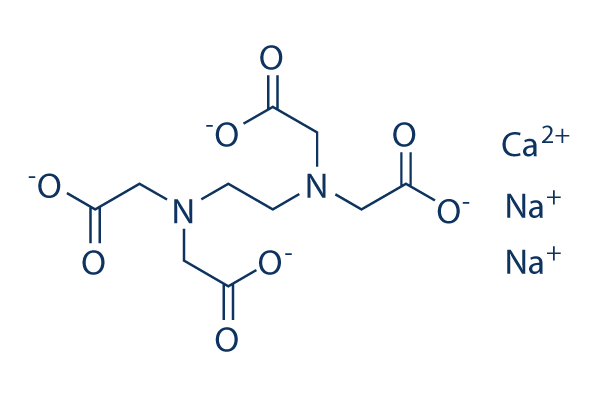
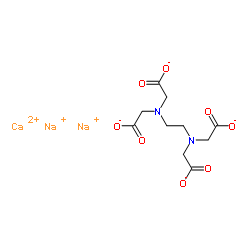
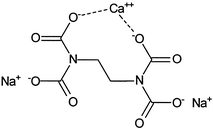
Structural Formula Of Calcium Disodium Edta Cana 2 Edta Download Scientific Diagram

139 33 3 Ethylenediaminetetraacetic Acid Disodium Salt Chelaplex Chelaplex Iii Chelaton Iii Chelest 0 Chelest 2b Sd Chelest 2bs Chelest B Clewat N Clewat N 2 Complexon Iii Disodium Ethylenedinitrilo Tetraacetate Disodium Edta Disodium
Q Tbn And9gcrgsoenyf5aizal0g2mxul 57ghgq7kbfurdfxnedpyybsitioh Usqp Cau

Disodium Edetate Disodium Edta Drug Molecule Skeletal Formula Royalty Free Cliparts Vectors And Stock Illustration Image

Simultaneous Determination Of Edta Sorbic Acid And Diclofenac Sodium In Pharmaceutical Preparations Using High Performance Liquid Chromatography Abstract Europe Pmc
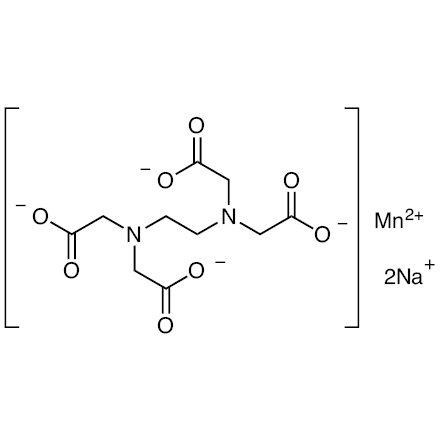
Ethylenediaminetetraacetic Acid Manganese Disodium Salt 84 5 Tci Shanghai Development Co Ltd

In Situ Modification Of Activated Carbon With Ethylenediaminetetraacetic Acid Disodium Salt During Phosphoric Acid Activation For Enhancement Of Nickel Removal Sciencedirect

Edta Openwetware
Www Teknoscienze Com Contents Riviste Pdf Af2 11 Rgb 46 51 Pdf
Edta Calcium Disodium 62 33 9
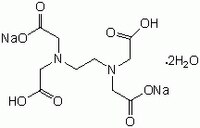
Edta Disodium Salt Dihydrate Molecular Biology Grade Cas 6381 92 6 Calbiochem Cas 6381 92 6
Calcium Edetate Calcium Disodium Edta Springerlink
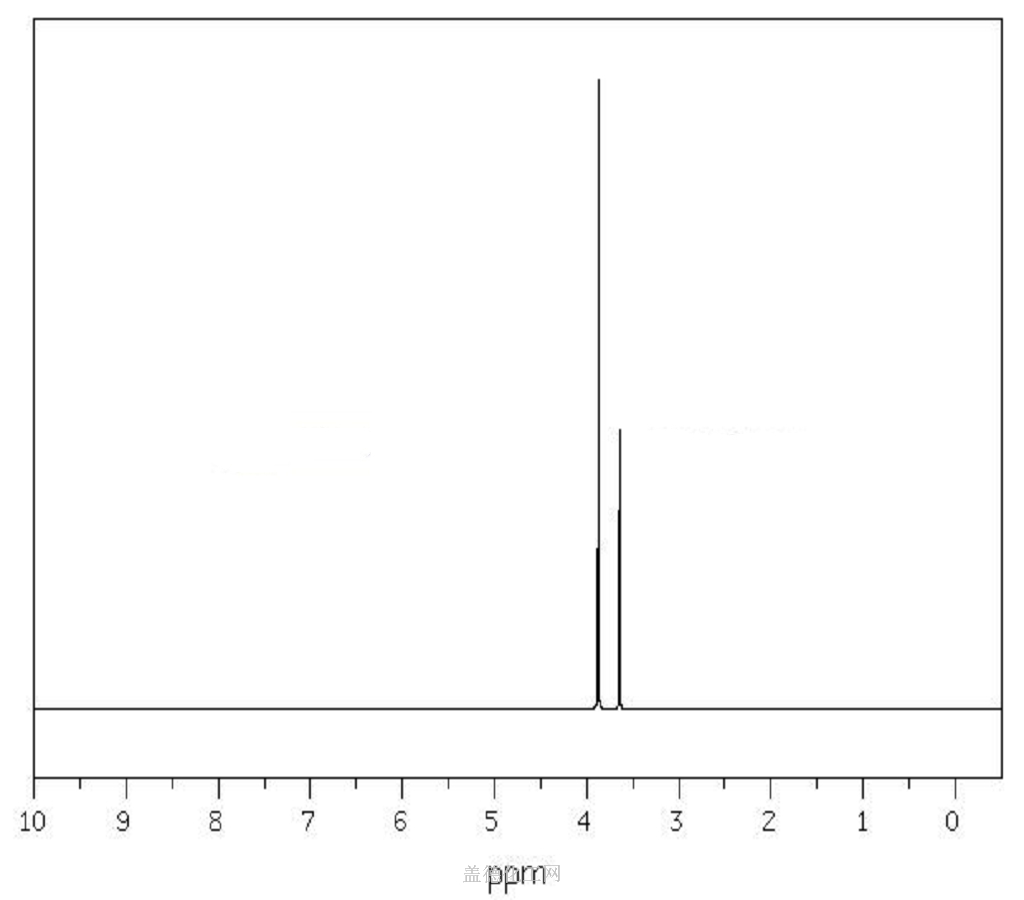
139 33 3 Edta Disodium Salt Anhydrous Formula Nmr Boiling Point Density Flash Point
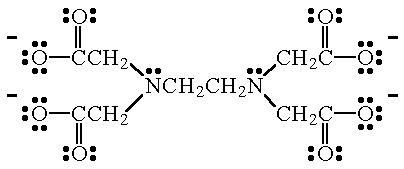
Coordination Compounds Help Page

Thermal Decomposition Of Ethylenediaminetetraacetic Acid In The Presence Of 1 2 Phenylenediamine And Hydrochloric Acid
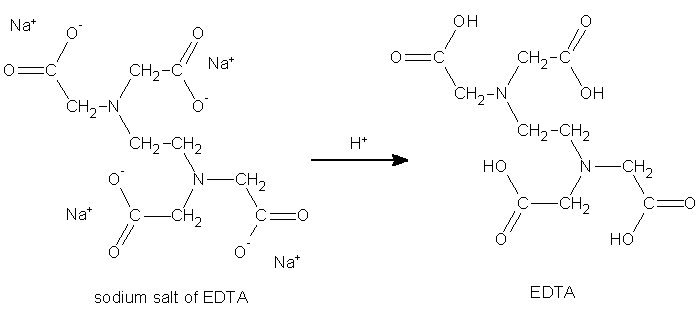
Synthesis Of Edta
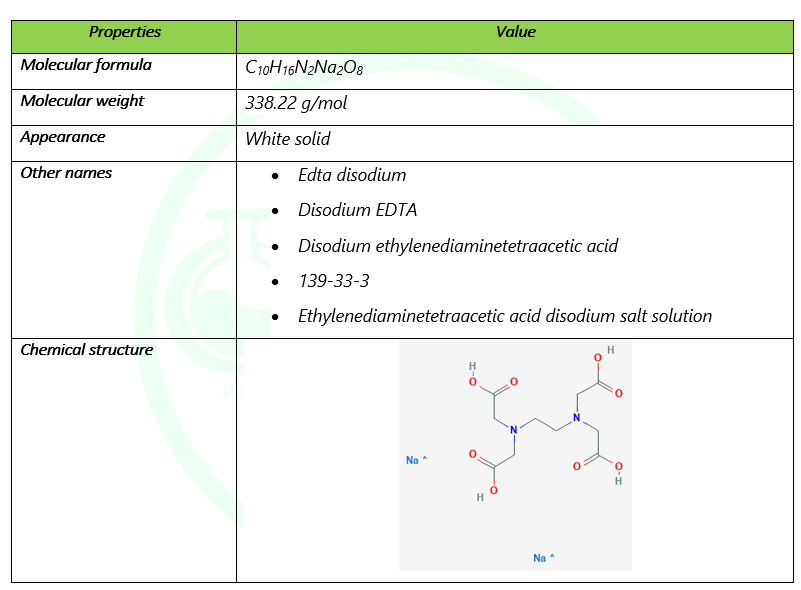
Edta 2na Shanghai Chemex Group Ltd

Structures Of Edta Disodium Salt Left And Metal Edta Complexes Right Download Scientific Diagram
Preparation Of Edta Solution Sharebiology
Disodium Edetate Dihydrate 6381 92 6

Disodium Edetate Dihydrate Supplier Casno 6381 92 6

Structural Formula Of Calcium Disodium Edta Cana 2 Edta Download Scientific Diagram
Ethylenediaminetetraacetic Acid 2 Solution 139 33 3 Sigma Aldrich
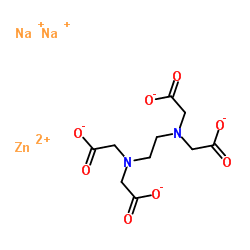
Zinc Disodium Edta Cas 21 9 Chemsrc
Edta Motm
Cas 78 1 Ethylenediaminetetraacetic Acid Calcium Disodium Salt Hydrate Chemsrc

Disodium Edta Molecule Illustration Stock Image F028 2597 Science Photo Library

Short Review Of Calcium Disodium Ethylene Diamine Tetra Acetic Acid As A Food Additive Semantic Scholar
Edta Disodium Salt Anhydrous 139 33 3 Wiki
Ethylenediaminetetraacetic Acid 99 1 Sigma Aldrich
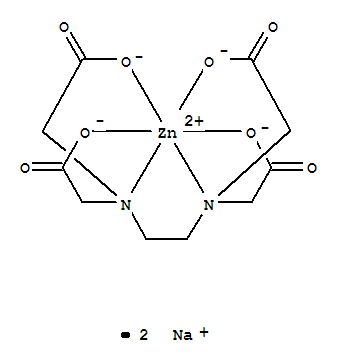
Zinc Disodium Edta Supplier Casno 21 9

What Is The Structural Formula For Edta Quora
Edta Disodium Salt C10h14n2na2o8 Chemspider

Ferric Sodium Edta Wikipedia

Disodium Edetate Dihydrate Supplier Casno 6381 92 6

Edta Disodium Zinc Salt Tetrahydrate Honeywell Research Chemicals
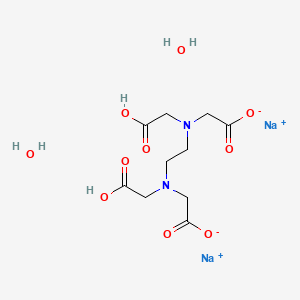
Disodium Edetate Dihydrate C10h18n2na2o10 Pubchem

Ethylenediaminetetraacetic Acid Wikiwand

Ethylenediaminetetraacetic Acid Edta Disodium Salt Solution 0 025 Mol L 0 05 N X 1 L Ac 6381 92 6 Scharlau Scharlab Com Scharlab S L The Lab Sourcing Group

Thermal Decomposition Of Ethylenediaminetetraacetic Acid In The Presence Of 1 2 Phenylenediamine And Hydrochloric Acid
Synthesis Of Edta

Using Disodium Edta As An Anionic Surfactant To Control Solute Solubility In Blood And Water Solutions

Endrate Edetate Uses Dosage Side Effects Interactions Warning
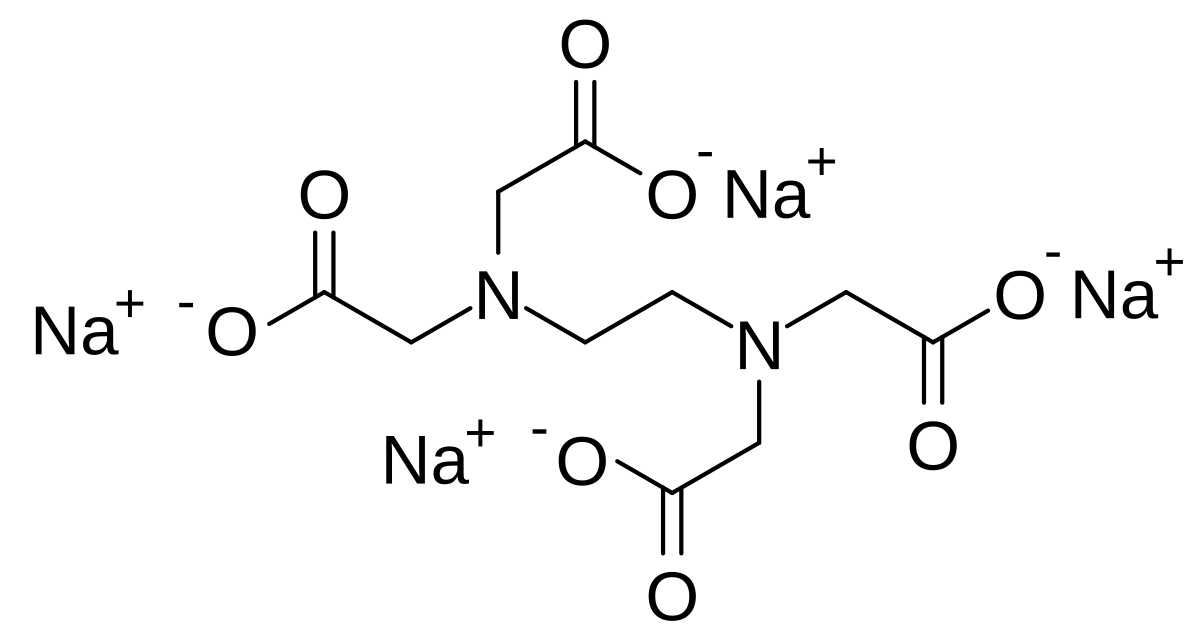
Tetrasodium Edta Wikipedia
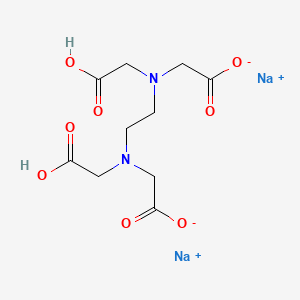
Edetate Disodium Drug Information Uses Side Effects Chemistry Pharmacompass Com
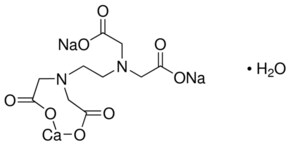
Ethylenediaminetetraacetic Acid 98 78 1 Sigma Aldrich
6 3 Complexometric Titrations ตำรายาของประเทศไทย กรมว ทยาศาสตร การแพทย
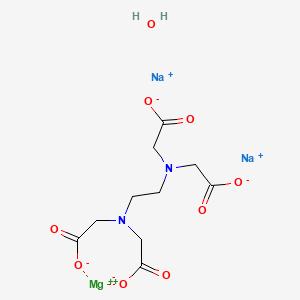
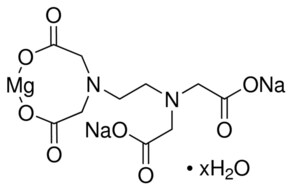
Magnesium Disodium Edta Tetrahydrate American Elements
Q Tbn And9gctvk0lldxwtanoeeq6 hoyavri6nkkumyiscinj5lvpm79wh6 Usqp Cau
Q Tbn And9gctvk0lldxwtanoeeq6 hoyavri6nkkumyiscinj5lvpm79wh6 Usqp Cau

4 Reasons Edta Is In Your Skincare And Cleansers The Dermatology Review
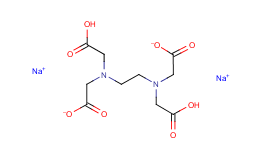
Edta 2na China Edta 2na Edta 2na Exporters China Edta 2na Suppliers Wyhuafeng
Edta Cameo

Calcium Disodium Edta Phar6157
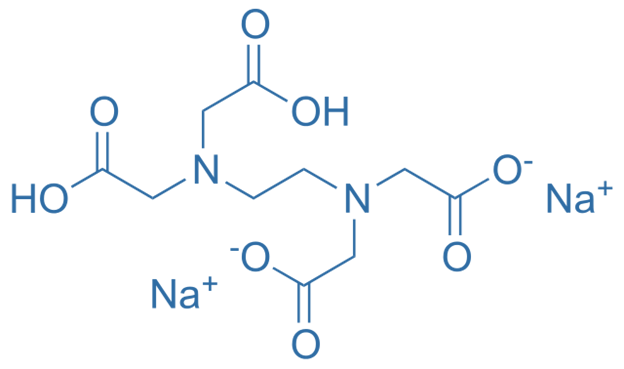
Difference Between Disodium Edta And Tetrasodium Edta Definition Structure Uses
2
Edathamil C10h12cun2na2o8 Chemspider
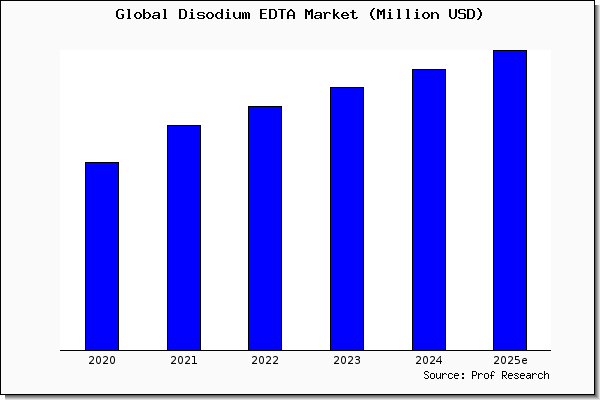
Disodium Edta Market Size Share Trend And Forcarst To 25 Prof Research

Honeywell Fluka Edta Disodium Salt Solution Volumetric 0 05 M Edta Na2 Honeywell Fluka 500ml Plastic Bottle Honeywell Fluka Edta Disodium Salt Solution Volumetric 0 05 M Edta Na2 Honeywell Fluka Fisher Scientific
Ethylenediaminetetraacetic Acid Wikipedia
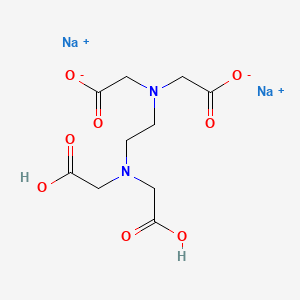
Edta Disodium Salt C10h14n2na2o8 Pubchem

Calcium Disodium Edta Phar6157
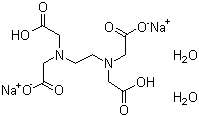
Ethylenediaminetetraacetic Acid Disodium Salt Edta 2na Cas 6381 92 6 Chemical Suppliers

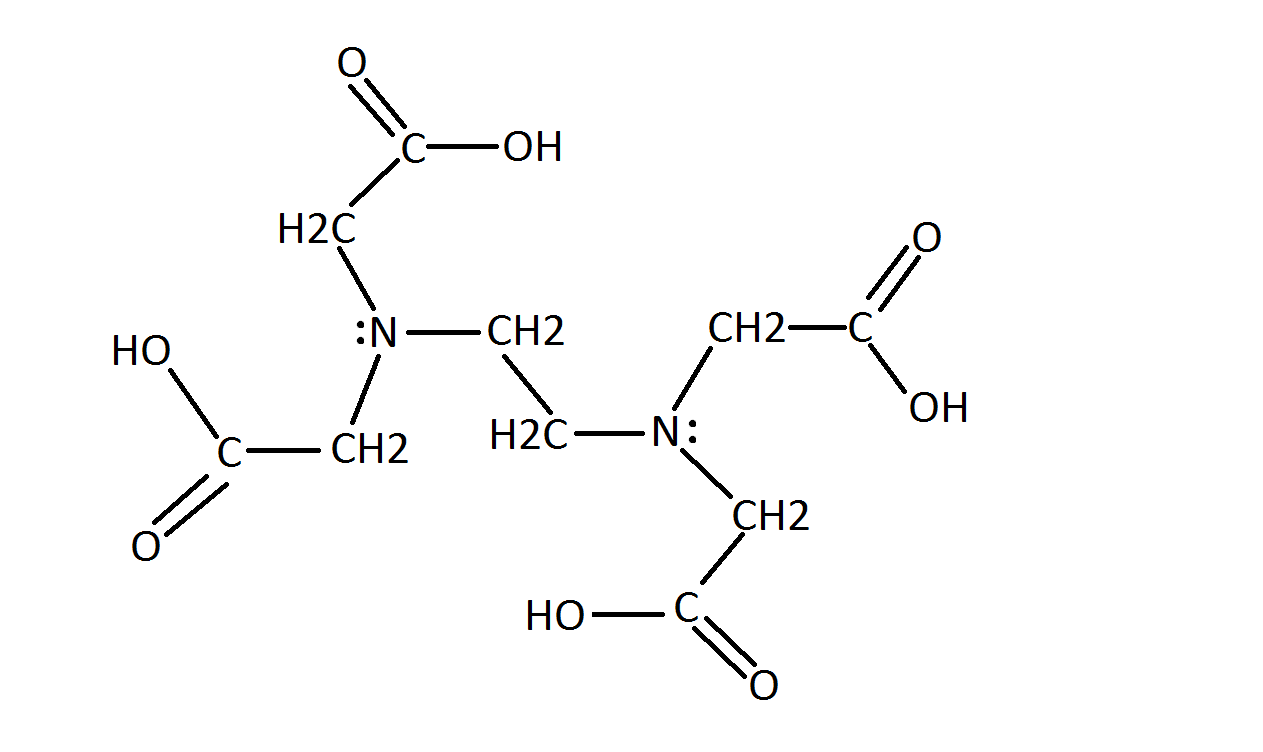
Structural Formula Of Disodium Edetate Download Scientific Diagram
Http Journals Sagepub Com Doi Pdf 10 1080

Calcium Disodium Versenate Medicis Pharmaceutical Corp Fda Package Insert
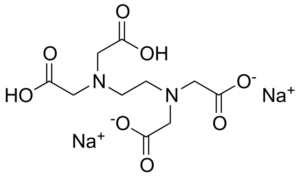
5 Top Difference Between Disodium Edta And Tetrasodium Edta With Table Core Differences

Edta Disodium Salt Solution With Zinc Complex Added Solution B 1 Ml Solution 1 German Degree Of Hardness In 100 Ml Of Water For Complexometry Honeywell Fluka Products Fisher Scientific
Edta 4na Shanghai Chemex Group Ltd

Dow Versene Food Grade Edta Product Article Chempoint
Edta Chemistry Libretexts

Comparative Evaluation Of Disodium Edetate And Diethylenetriaminepentaacetic Acid As Iron Chelators To Prevent Metal Catalyzed Destabilization Of A Therapeutic Monoclonal Antibody Journal Of Pharmaceutical Sciences

Ethylenediaminetetraacetic Acid An Overview Sciencedirect Topics
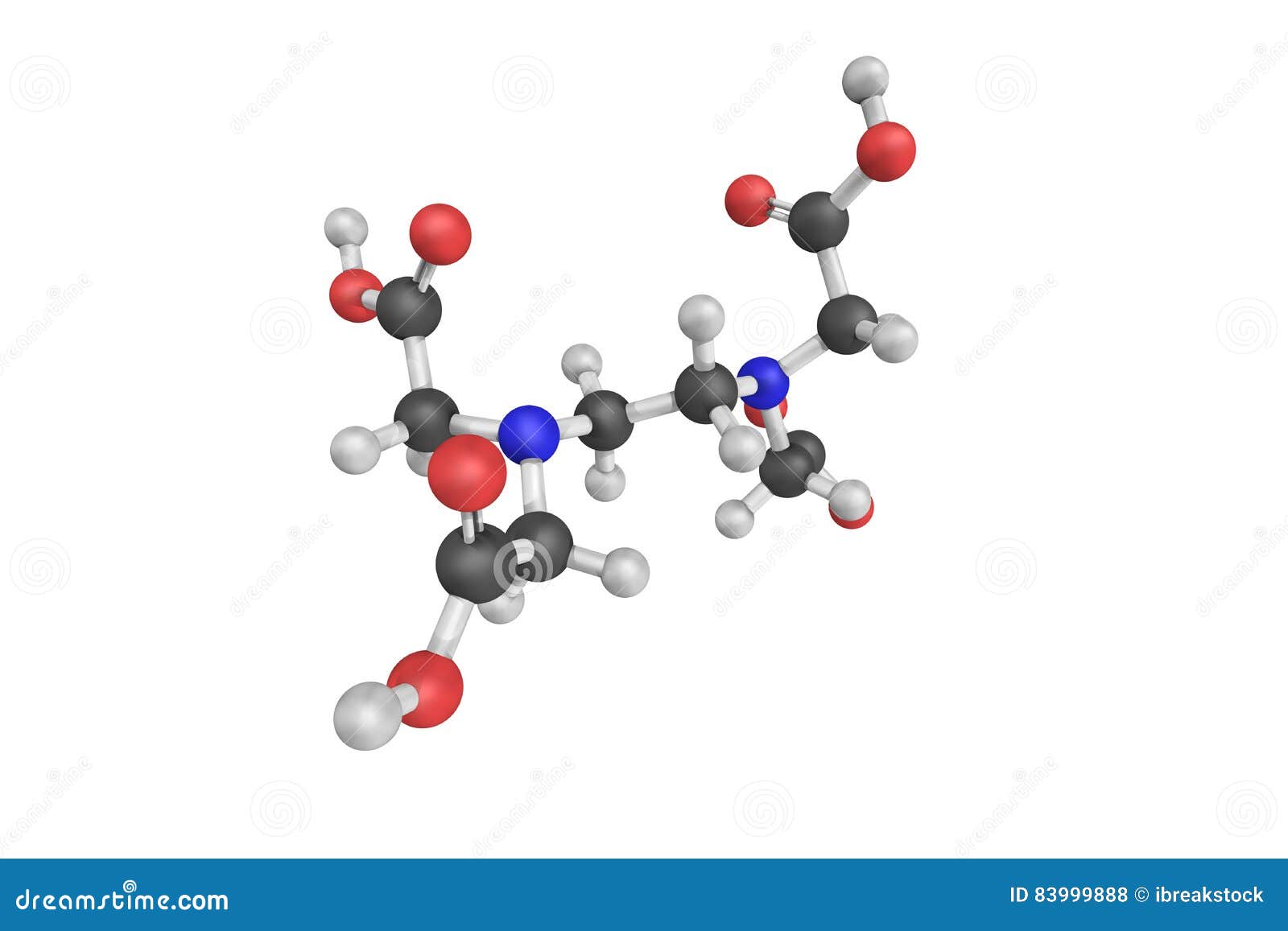
3d Structure Of Edtacal Also Known As Calcium Disodium Edta Stock Illustration Illustration Of Sodium Calcium 9998
Ethylenediaminetetraacetic Acid 99 1 Sigma Aldrich
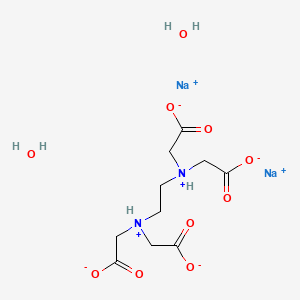
Arma Food Pharma
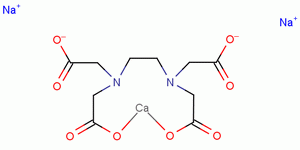
Edta Ca 62 33 9 China Edta Ca 62 33 9 Manufacturers China Edta Ca 62 33 9 Suppliers
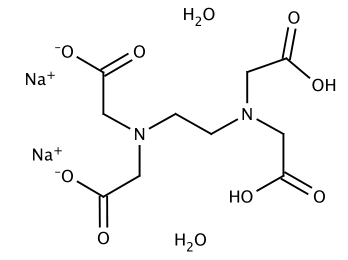
Disodium Edta Dihydrate C10h18n2na2o10 Pubchem
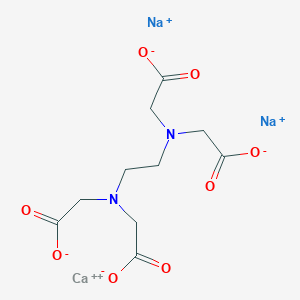
62 33 9 Drug Information Uses Side Effects Chemistry Pharmacompass Com
Sodium Calcium Edetate Wikipedia

Edta Calcium Disodium Salt 62 33 9 Carbosynth Product

Calcium Disodium Edta C10h14can2na2o8 2 Pubchem
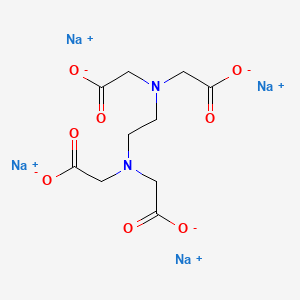
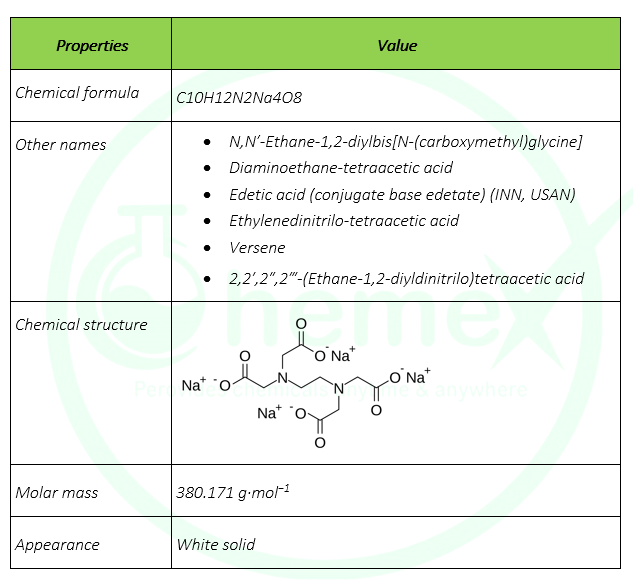
Edetate Sodium C10h12n2na4o8 Pubchem
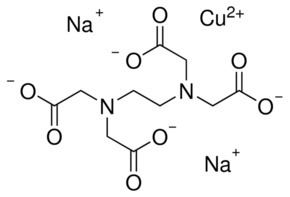
Ethylenediaminetetraacetic Acid Alchetron The Free Social Encyclopedia

Edta Disodium Salt Dihydrate Honeywell Research Chemicals

Chelation Therapy In Medicinal Chemistry Pharmafactz



