Disodium Edta Full Form
Disodium Edta Dihydrate C10h18n2na2o10 Chemspider

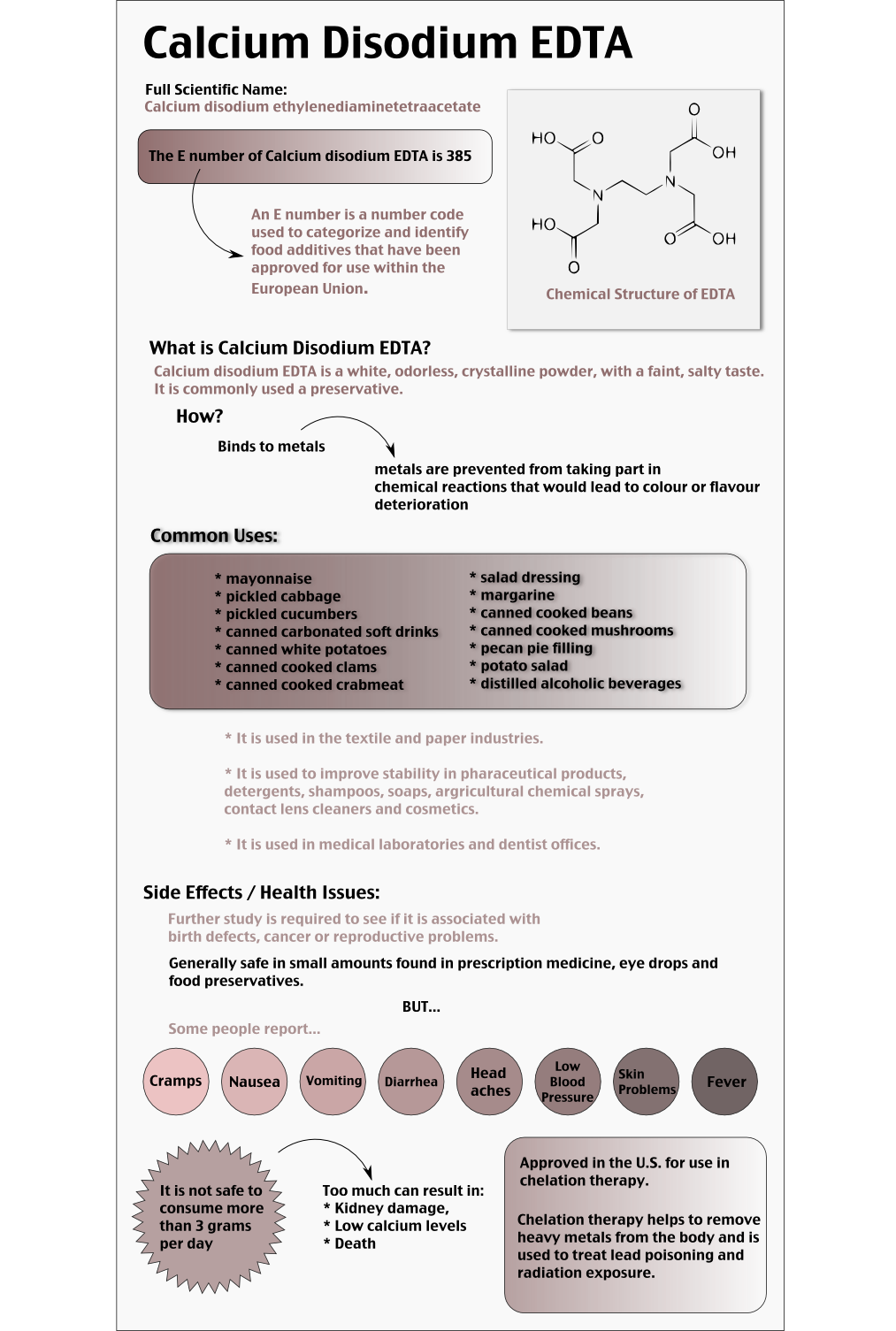
Is Calcium Disodium Edta Bad For You Here Is Your Answer
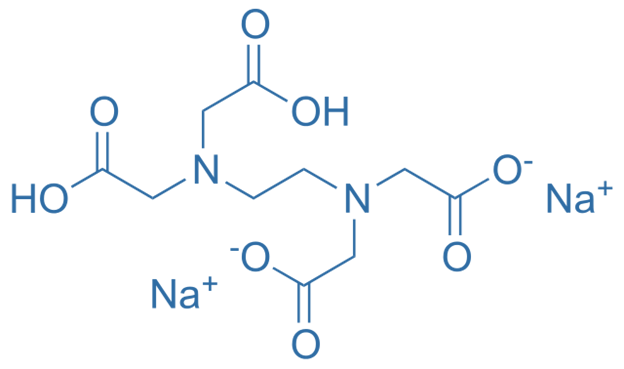
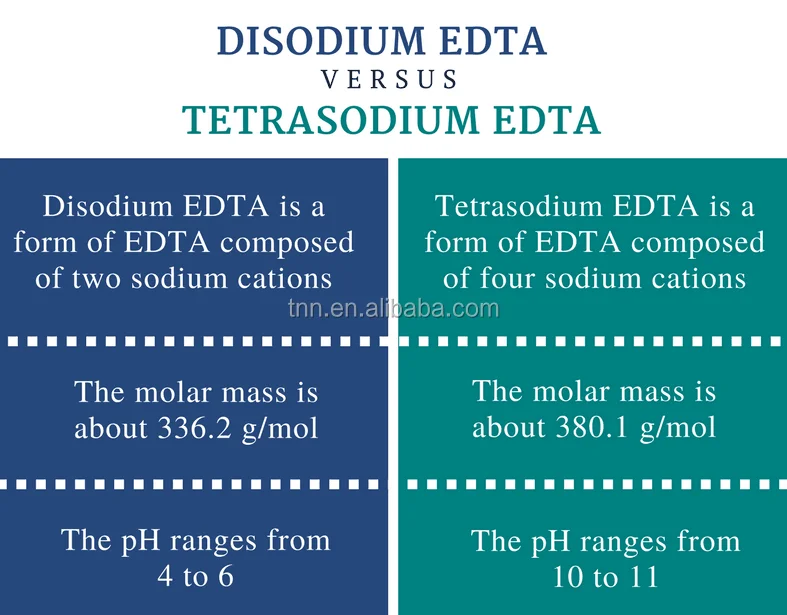
Difference Between Disodium Edta And Tetrasodium Edta Definition Structure Uses
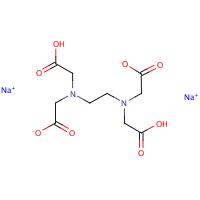
Disodium Edta Dihydrate Hazardous Agents Haz Map

Disodium Edetate Injection Usp Sgpharma

Dissolvine Na2 P Disodium Edta Tinnakorn
EDTA, disodium, dihydrate Ethylene diamine tetraacetic acid, disodium salt Glycine, N,N'1,2ethanediylbisN(carboxymethyl), disodium salt, dihydrate Disodium EDTA About Disodium EDTA Enquire Now Our team of experts are at the ready Fill out the form below and we will be in touch shortly * *.
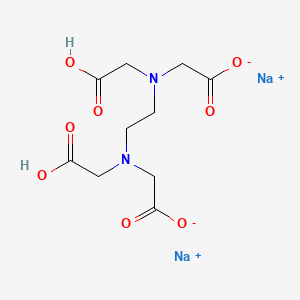
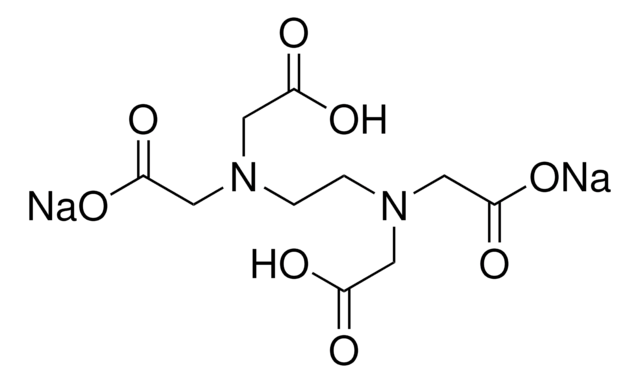
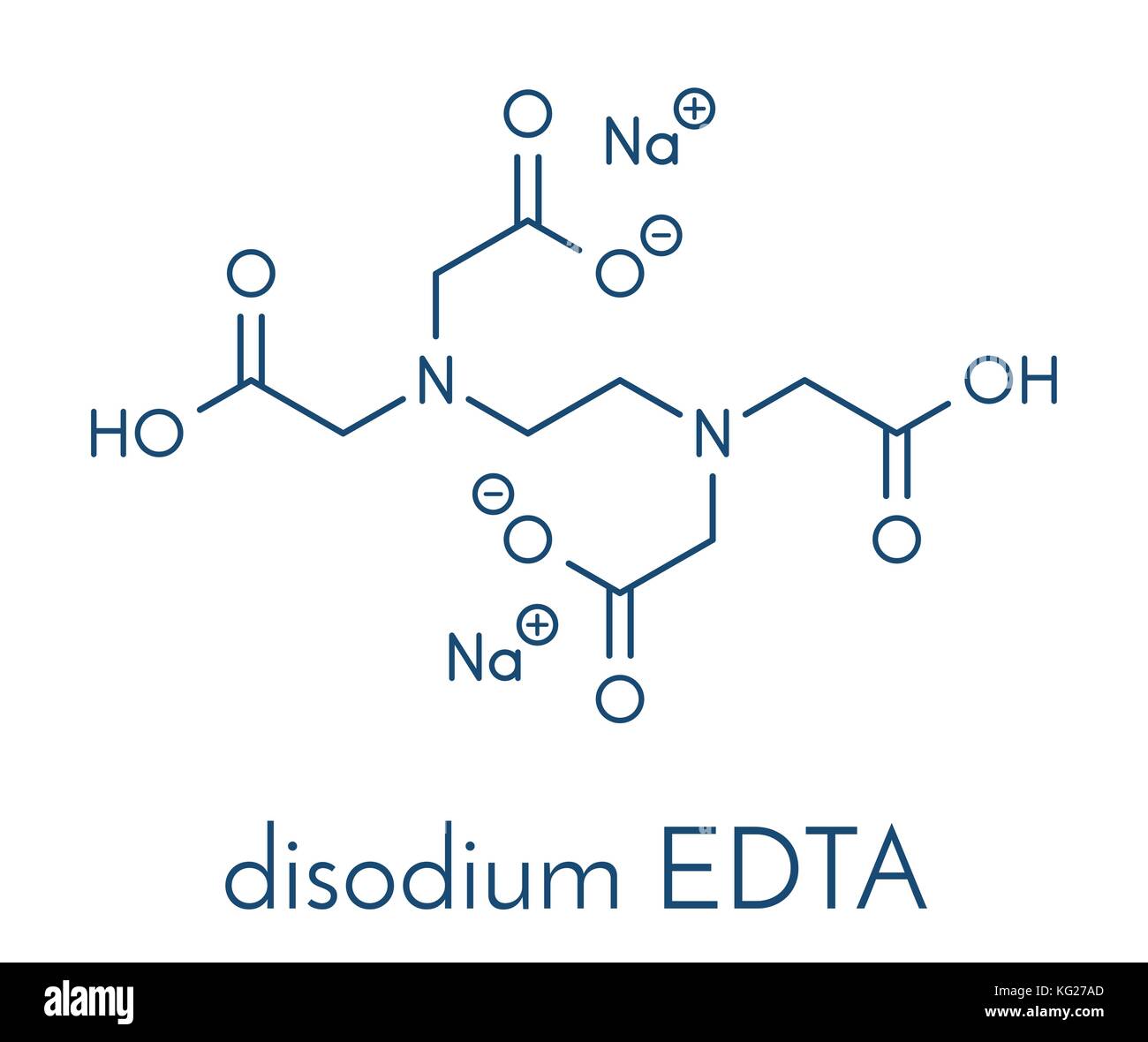
Disodium edta full form. Edetate disodium (EDTA) is a chelating (KEElateing) agent A chelating agent is capable of removing a heavy metal, such as lead or mercury, from the blood. Product form Substance Substance name Disodium EDTA Formula C 10 H 14 N 2 Na 2 O 8 Molecular weight g/mol CAS No Product code LWEDTA Synonyms Ethylenediaminetetraacetic acid disodium salt dihydrate 12 Relevant identified uses of the substance or mixture and uses advised against. EDTA (ethylenediaminetetraacetic acid) is a chelating agent This is a characteristic of compounds which can capture atoms that otherwise precipitate or form scales on other materials.
Page 1 of 11 Safety Data Sheet Section 1 Identification Product Name EDTA (17% Solution) Product Use Root canal chelating conditioner and cleanser Manufacturer InterMed, Inc / Vista Dental Products Address 20 South St Suite A, Racine, WI Phone (877) Fax (262) 24 HR Emergency Telephone Number CHEMTREC (North America). What is edetate disodium?. EDTA or ethylenediaminetetraacetic acid is a common ingredient in skincare and body care products EDTA helps to improve the foaming of cleansers, soaps, and body washes Two primary forms of EDTA are frequently used in personal care products, Tetrasodium EDTA and Disodium EDTA The main difference between tetrasodium EDTA and disodium EDTA.
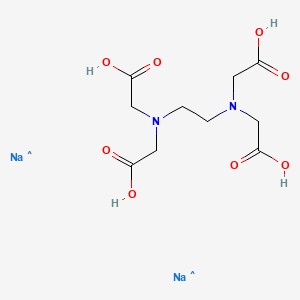
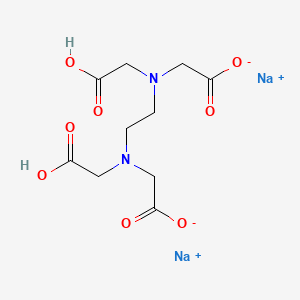
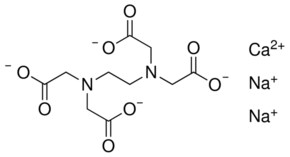
Calcium disodium ethylenediaminetetraacetic acid (EDTA) is a chelating agent that can bind to certain trace elements and render them inactive 1 Because of its ability to remove unwanted metals, it has been used intravenously in clinical settings for several decades Oral chelation therapy with EDTA uses the same type of EDTA as used by intravenous therapy practitioners. Disodium EDTA The chemical formula of disodium EDTA is C 10 H 14 N 2 Na 2 O 8 Tetrasodium EDTA The chemical formula of tetrasodium EDTA is C 10 H 14 N 2 Na 4 O 8 Molar Mass Disodium EDTA The molar mass of disodium EDTA is about 3362 g/mol Tetrasodium EDTA The molar mass of tetrasodium EDTA is about 3801 g/mol Normal pH. EDTA stands for Ethylene Diamine Tetra Acetic acid As it is insoluble in water, we use its disodium salt Structure of EDTA By nature, Eriochrome Black T indicator is blue in colour When EBT indicator is added to water sample, it forms a wine red coloured unstable CaMgEBT complex.
Edetate disodium is a chelating agent with high affinity for lead and cadmium (7) The Trial to Assess Chelation Therapy (TACT) demonstrated that edetate disodium–based chelation reduced cardiovascular events, especially in patients with diabetes after myocardial infarction (8,9). EDTA helps to improve the foaming of cleansers, soaps, and body washes Two primary forms of EDTA are frequently used in personal care products, Tetrasodium EDTA and Disodium EDTA The main difference between tetrasodium EDTA and disodium EDTA is the structure of the molecules and the pH. EDTA, also known as disodium EDTA, EDTA disodium, or ethylenediaminetetraacetic acid, is a widely used chemical compound found in personal care, skin care, processed foods, cosmetic preparations,.
005–011–01–1 and 005–011–02–9), CLH information cannot be displayed in the InfoCard as the difference between the CLH classifications requires manual interpretation or. CAS# , EDTA, Ethylenediaminetetraacetic acid is a molecule which complexes metal ions in aqueous environments It is available in four neutralizations, two of which, Disodium EDTA and Tetrasodium EDTA, are commonly used in the cosmetics Generally, the choice of which product to use is determined by the pH of your product. EDTA stands for Ethylene Diamine Tetra Acetic acid As it is insoluble in water, we use its disodium salt Structure of EDTA By nature, Eriochrome Black T indicator is blue in colour When EBT indicator is added to water sample, it forms a wine red coloured unstable CaMgEBT complex.
The disodium form of EDTA is approved by the FDA for this use, but healthcare providers generally prefer other treatments such as lidocaine or phenytoin (Dilantin) These treatments are considered. Calcium disodium EDTA is found in food, personal care products, cosmetic and industrial productions EDTA is also used for chelation therapy of heavy metals and toxins “Calcium Disodium – EDTA is also used to help preserve food;. Disodium EDTA has the chemical formula of CH2N (CH2CO2H)22, the molecular formula of C10H16N2O8, and is a major chelating agent from which all of its applications extend from It has high.
The full form of EDTA is Ethylenediaminetetraacetic acid It is an amino acid compound and acts as a chelating agent to remove the metals naturally from the body Various types of EDTA products are manufactured and available in the market for different industrial purpose. EDTA in its disodium salt or calcium disodium salt form is frequently used in pharmaceuticals because of its stability, compatibility and low toxicity In the field of analytical chemistry, besides its use in complexometric titrations, EDTA has been reported to be very useful ligands for the complexation of metals, which enables their. Calcium disodium ethylenediaminetetraacetic acid (EDTA) is a chelating agent that can bind to certain trace elements and render them inactive 1 Because of its ability to remove unwanted metals, it has been used intravenously in clinical settings for several decades Oral chelation therapy with EDTA uses the same type of EDTA as used by intravenous therapy practitioners.
EDTA is a molecule called a chelating agent A chelating agent is a clawlike substance that can grab and stick to other molecules Some types of EDTA stick to calciumOther types stick to metals. Similarly, regular blood donors show decreased incidence of myocardial infarction and cancer, recalling the Swiss experience with regular EDTA rapid IV injections of Calcium Disodium EDTA in carefully monitored patients over almost two decades For example, the level of lead has now been shown to relate directly to IQ106. The chelating agent edetate disodium (EDTA) disrupts biofilm which are harboring bacteria and fungi in the nasal passages Disrupting the biofilm is an important step in several protocols for treating CIRS related to infections caused by mold exposure Xylitol can be added to EDTA to provide more effective cleansing of the nasal passages.
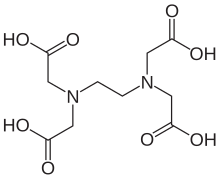
If the substance is covered by more than one CLH entry (eg disodium tetraborate EC no 215–540–4, is covered by three harmonisations:. Ethylenediaminetetraacetic acid ( EDTA) is an aminopolycarboxylic acid with the formula CH 2 N (CH 2 CO 2 H) 2 2 This white, watersoluble solid is widely used to bind to iron and calcium ions It binds these ions as a hexadentate ("sixtoothed") chelating agent. Edetate disodium Generic Name edetate disodium (EDTA) (E de tate dye SOE dee um) Brand Name Disotate, Endrate, Meritate Uses;.
Throughout the study, we used the tetrasodium salt form, as it has been shown to have a better range of activity than the disodium form of EDTA on biofilm formation in other biofilm models ELISA plates were initially coated with MAb 18, as it was shown previously to aid in initial biofilm formation and thus provide results faster than with. The disodium form of EDTA is approved by the FDA for this use, but healthcare providers generally prefer other treatments such as lidocaine or phenytoin (Dilantin) These treatments are considered. Disodium ethylenediaminetetraacetic acid (EDTA) EDTA combines with polyvalent cations, including calcium ions, to form a soluble nonionic complex that can be excreted It requires IV administration and is usually administered two or more times a week.
Edetate disodium anhydrous is a polyvalent ion chelator that reduces blood concentrations of calcium or digitalis 2,3 It has a long duration of action as patients are generally given 1 daily dose 2,3 The therapeutic index is wide, as high doses are generally well tolerated 2,3 Patients should be counselled regarding the risk of postural hypotension, effects of myocardial contractility, hypokalemia, hypomagnesemia, and hypoglycemia 2,3. What Is Calcium Disodium (EDTA) Used For?. Disodium EDTA is a chemical that is used as a food preservative that is commonly found in salad dressings, sauces, spreads, canned goods, and beverages It is also present as a preservative in soaps, shampoos, and cosmetics It is both odorless and colorless and works to preserve foods and creams by binding with various minerals and metals.
EDTA stands for ethylenediaminetetraacetic acid, chemical formula C10H16N2O8 It is synthesised in a lab, from ethylenediamine, formaldehyde, and sodium cyanide Disodium EDTA is the salt produced as a result It is a metal chelation agent, which means it binds with and deactivates heavy metal ions. EDTA (Disodium EDTA) 210mg ** Lipoic Acid (from Sodium RLipoate) 26mg ** Phosphatidylcholine (from purified soybean lecithin) 450mg ** **Daily Value (DV) not established Other Ingredients Glycerin, Ethanol, Water, Sodium Hydroxide. EDTA is a polyamino carboxylic acid with the formula CH2N(CH2CO2H)22 This colourless, watersoluble solid is widely used to dissolve scale Its usefulness arises because of its role as a.
Disodium ethylenediaminetetraacetic acid (EDTA) EDTA combines with polyvalent cations, including calcium ions, to form a soluble nonionic complex that can be excreted It requires IV administration and is usually administered two or more times a week. Product form Substance Substance name Disodium EDTA Formula C 10 H 14 N 2 Na 2 O 8 Molecular weight g/mol CAS No Product code LWEDTA Synonyms Ethylenediaminetetraacetic acid disodium salt dihydrate 12 Relevant identified uses of the substance or mixture and uses advised against. Product form Substance Substance name Disodium EDTA Formula C 10 H 14 N 2 Na 2 O 8 Molecular weight g/mol CAS No Product code LWEDTA Synonyms Ethylenediaminetetraacetic acid disodium salt dihydrate 12 Relevant identified uses of the substance or mixture and uses advised against.
Detoxamin EDTA Suppositories are one the best means to improve your overall health With glutathione or without, for even more detoxification, antioxidant and immune support, increased energy, body repair and support The EDTA supplement that gets right to where the metals are stored for deep EDTA metal chelation Get all your questions answered now!. Mg vials and each cc contains 300 mg (twice as concentrated as the old Disodium EDTA we have used in the past) This is given through a 23gauge butterfly infusion needle and usually we simply take an empty disposable 10 cc plastic syringe and give the CALCIUM EDTA directly in the vein with this syringe without any dilution. Edetate Disodium (sodium EDTA) is a chelating agent that is used in the treatment of corneal ulcers Keratomalacia, or corneal melting, is the rapid degeneration of collagen and other components of the stoma of the cornea, which can lead to perforation of the cornea.
EDTA disodium salt C10H14N2Na2O8 CID 1300 structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Disodium EDTA C10H16N2Na2O8 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Chelating agent is a partially neutralized salt of EDTA, in dry form, and is suitable for direct food applications It is well suited for applications calling for neutral pH or mildly acidic conditions common to most food and pharmaceutical products.
EDTA, or ethylenediaminetetraacetic acid, is a popular organic molecule that acts as a chelating agent in food, chelation therapy and in many household products EDTA calcium disodium a food preservative and food stabilizer that also treats lead poisoning and hypercalcemia. Definition EDTA (Ethylenediaminetetraacetic acid) is a transparent, watersoluble compound first synthesized in 1935 It belongs to a class of molecules called chelators (from the Greek word chelé, meaning “claw”) that bind – chelate – metals 1. I want to chelate metal ion using EDTA I saw in blood tube for anticoagulant, they use K2EDTA But I have disodium salt EDTA in my lab (mw g/mol) So, which one is better?.
Mg vials and each cc contains 300 mg (twice as concentrated as the old Disodium EDTA we have used in the past) This is given through a 23gauge butterfly infusion needle and usually we simply take an empty disposable 10 cc plastic syringe and give the CALCIUM EDTA directly in the vein with this syringe without any dilution. The second study of oral Ca EDTA disodium was published in 1954, using a daily dose of 2 grams for 7 days (4) In the symptomatic patients with lead intoxication, the symptoms improved remarkably following oral EDTA and the blood profile returned to normal. Throughout the study, we used the tetrasodium salt form, as it has been shown to have a better range of activity than the disodium form of EDTA on biofilm formation in other biofilm models ELISA plates were initially coated with MAb 18, as it was shown previously to aid in initial biofilm formation and thus provide results faster than with.
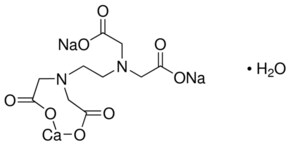
What Is Calcium Disodium (EDTA) Used For?. Disodium EDTA is a form of EDTA with two sodium cations It is a heavy metal chelating agent and present as a dry powder The general structure of EDTA contains four negatively charged oxygen atoms Out of the four, two oxygen atoms of EDTA remain combined with two sodium cations to form disodium EDTA Therefore, disodium EDTA is a synthesized byproduct of EDTA. Calcium disodium EDTA is approved by the FDA for use in lead poisoning and has been the mainstay of treatment for childhood lead poisoning since the 1950s 4 The second drug, disodium EDTA, is approved for use in patients with rhythm disorders from drug intoxication such as digitalis where there is hypercalcemia Calcium disodium EDTA.
EDTA helps to improve the foaming of cleansers, soaps, and body washes Two primary forms of EDTA are frequently used in personal care products, Tetrasodium EDTA and Disodium EDTA The main difference between tetrasodium EDTA and disodium EDTA is the structure of the molecules and the pH. EDTA stands for Ethylenediaminetetraacetic acid first synthesised by Ferdinand Münz in 1935 It's chemical formula is C10H16N2O8 and it's structure is given below It's conjugate base is Ethylenediaminetetraacetate which is a hexdendate ligand 34K views. The ideal form of oral EDTA would be the dipotassium salt of the magnesium chelate, since these two very important intracellular minerals would be dissociated in the intestinal tract and available for absorption The affinity of EDTA for magnesium is very low, resulting in exchange of magnesium for toxic metals in the intestinal tract.
Categories Miscellaneous EDTA is short for ethylenediamine tetraacetic acid, a stabilizer used in cosmetics to prevent ingredients in a given formula from binding with trace elements (particularly minerals) that can be present in water EDTA also keeps other ingredients from causing unwanted changes to a product’s texture, odor, and/or consistency. And to promote the color, texture, flavor of food and drink products. EDTA (Disodium) *Does not apply to reactions with Aluminum or other trivalent cations EDTA is the commonly used abbreviation for (Ethylenedinitrilo)tetraacetic Acid (also called Ethylenediaminetetraacetic Acid or Edetic Acid).
And to promote the color, texture, flavor of food and drink products. Other Names Disodium EDTA EDTANa2 Na2EDTA Disodium dihydrogen ethylenediamineN,N,N',N'tetraacetate, dihydrate EDTA, disodium, dihydrate Ethylene diamine tetraacetic acid, disodium salt Glycine, N,N'1,2ethanediylbis N (carboxymethyl), disodium salt, dihydrate. EDTA disodium is a chelating agent, which is a molecule that can create different bonds with one metal ion Once a chelating agent binds to the metal, it stabilizes it and the metal becomes water soluble so the body can eliminate toxic metals from the bloodstream and urine ( x, x ).
Ethylenediaminetetraacetic Acid Wikipedia
Pdf A Validated Reverse Phase Hplc Method For The Determination Of Disodium Edta In Meropenem Drug Substance With Uv Detection Using Precolumn Derivatization Technique

Disodium Edta Molecule Illustration Stock Image F028 2597 Science Photo Library

Ethylenediaminetetraacetic Acid Edta Disodium Salt Dihydrate Agr Labmaterials By Blanc Labo Sa
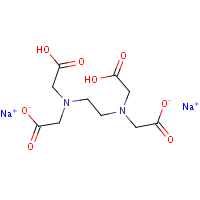
Disodium Edta C10h16n2na2o8 Pubchem
Edta Buy Edta Edta Price Disodium Edta Product On Alibaba Com
What Is Calcium Disodium Edta

Difference Between Disodium Edta And Tetrasodium Edta Compare The Difference Between Similar Terms

Disodium Edta Harmful Chemical To Avoid In Skin Care Metapora Metapora

Edta Disodium C10h14n2na2o8 Pubchem
Ethylenediaminetetraacetic Acid 2 Solution 139 33 3 Sigma Aldrich
Disodium Edetate Disodium Edta Drug Molecule Skeletal Formula Stock Vector Image Art Alamy
Edta Disodium Salt C10h14n2na2o8 Chemspider
Ethylenediaminetetraacetic Acid Wikipedia
Edta Cameo
Edta Motm

Edta Disodium Ip Bp Ep Usp Lab Chemicals प रय गश ल रस यन In Karchiya Vadodara Oasis Fine Chem Id

Difference Between Disodium Edta And Tetrasodium Edta Compare The Difference Between Similar Terms

Calcium Disodium Edta C10h14can2na2o8 2 Pubchem
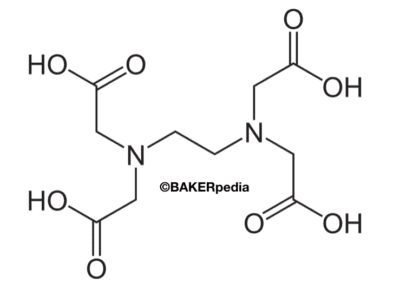
Ethlenediaminetetraacetic Acid Edta Baking Ingredients Bakerpedia

Solved 2 Calcium Disodium Versenate See Structure Below Chegg Com
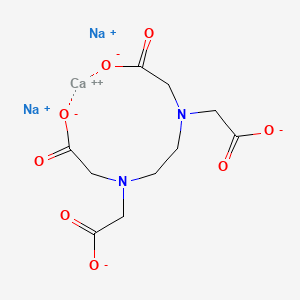
Calcium Disodium Ethylenediaminetetraacetate C10h12can2na2o8 Pubchem

Disodium Edta Msds Number Vs 01 Issue Date October
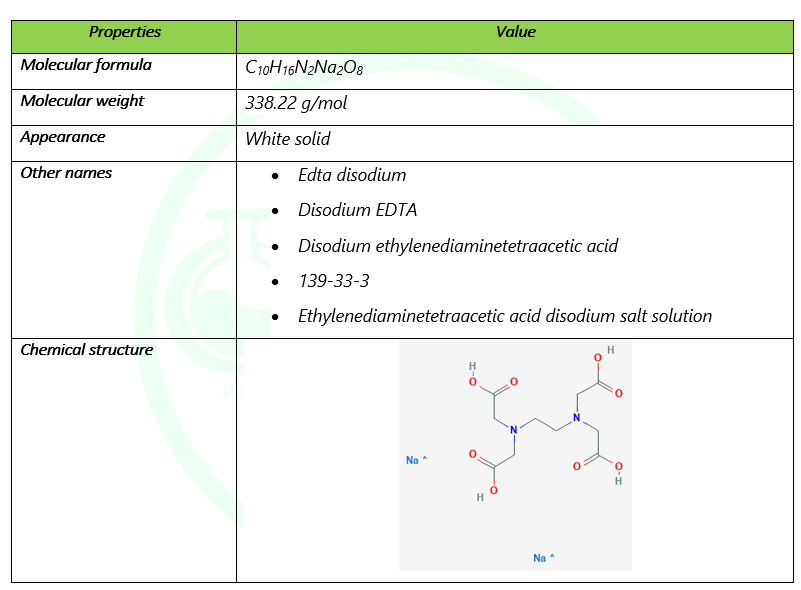
Edta 2na Shanghai Chemex Group Ltd
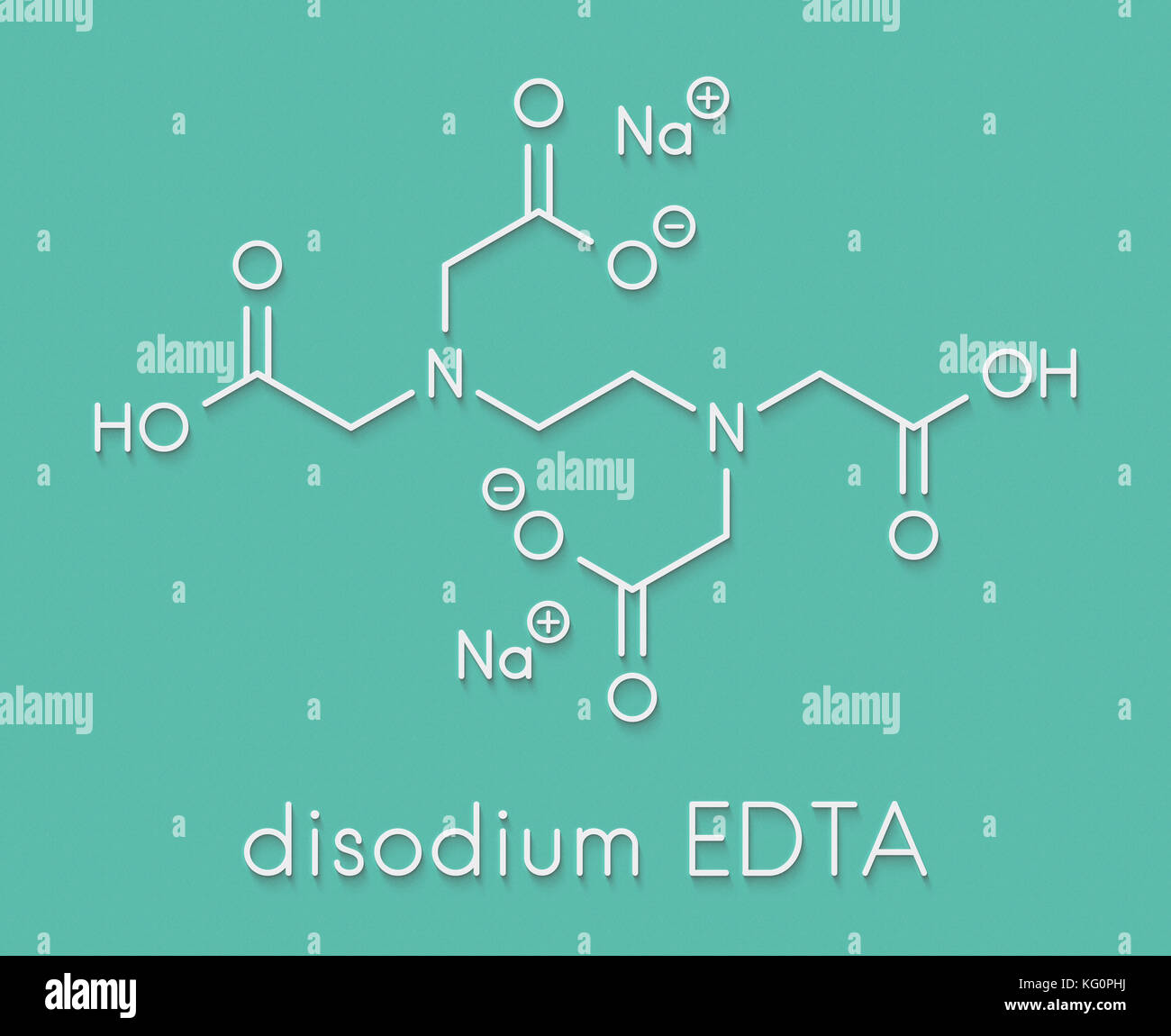
Edta High Resolution Stock Photography And Images Alamy
Edetate Disodium Drug Information Uses Side Effects Chemistry Pharmacompass Com

Calcium Disodium Edta Applications Safety And Side Effects

Ethylenediaminetetraacetic Acid Wikipedia

Calcium Disodium Edta In Wuse Meals Drinks Azuka Promise Chidi Jiji Ng
Sodium Calcium Edetate Wikipedia
Edetate Calcium Disodium Sigma Aldrich

Disodium Edta What You Need To Know
Course Instructor Muktadir S Hossain Msh3 Fall Ppt Download

Edta Disodium Salt Dihydrate Honeywell Research Chemicals

Data Analysis Standardizing The Disodium Edta Solu Chegg Com

Calcium Disodium Edetate Injection Usp 1000mg 5ml Ledcure Gnh India Exporter Distributor Wholesaler Comparators And Rld Supplier From India
Sodium Calcium Edetate European Pharmacopoeia Ep Reference Standard 62 33 9 Sigma Aldrich
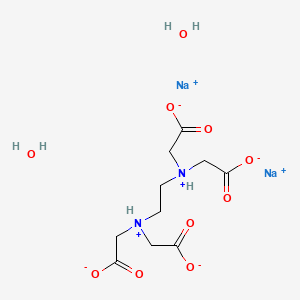
Disodium Edta Dihydrate C10h18n2na2o10 Pubchem

Edta Na2 Edta Disodium Salt Dihydrate Anhydrous

Solved Procedure 1 Prepare About 500 Ml Of Approximately Chegg Com

Spectrum E1001 125gm Disodium Edta Dihydrate Fcc Grade C10h14n2na2o8 2h2o 1 Cc Amazon Com Industrial Scientific

4 Reasons Edta Is In Your Skincare And Cleansers The Dermatology Review

Edta Calcium Disodium Calcium Disodium Edta Latest Price Manufacturers Suppliers

Honeywell Fluka Edta Disodium Salt Solution Volumetric 0 05 M Edta Na2 Honeywell Fluka 500ml Plastic Bottle Honeywell Fluka Edta Disodium Salt Solution Volumetric 0 05 M Edta Na2 Honeywell Fluka Fisher Scientific

Can Someone Explain Why Edta Needs Basic Condition For Dissolving
What Is The Structural Formula For Edta Quora

A Typical Chromatogram For The Mixture Of Disodium Edta Sorbic Acid Download Scientific Diagram

Edetate Disodium Edta For Chelations Injectable 30 Ml Preservative Free 150mg Ml Long Living Life

Edta Drops Edge Pharma

Short Review Of Calcium Disodium Ethylene Diamine Tetra Acetic Acid As A Food Additive Semantic Scholar
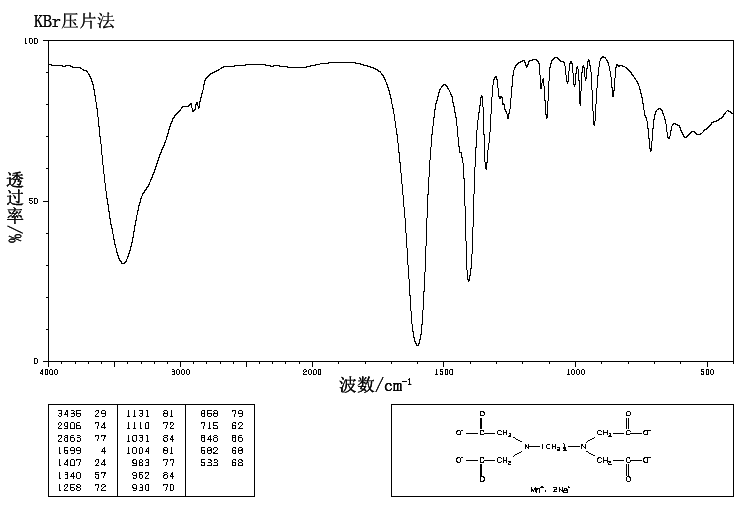
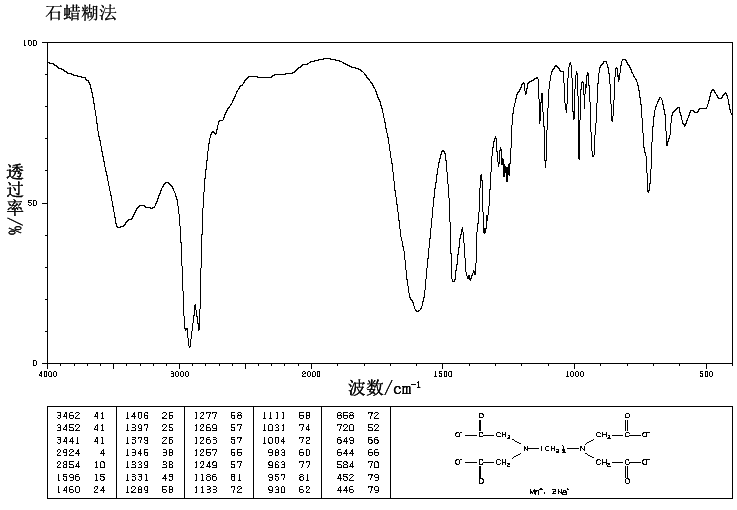
Manganese Disodium Edta Trihydrate 84 5 Ir2

Structural Formula Of Calcium Disodium Edta Cana 2 Edta Download Scientific Diagram
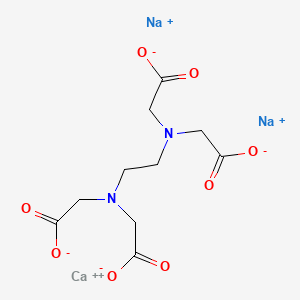
Edetate Calcium Disodium C10h12can2na2o8 Pubchem
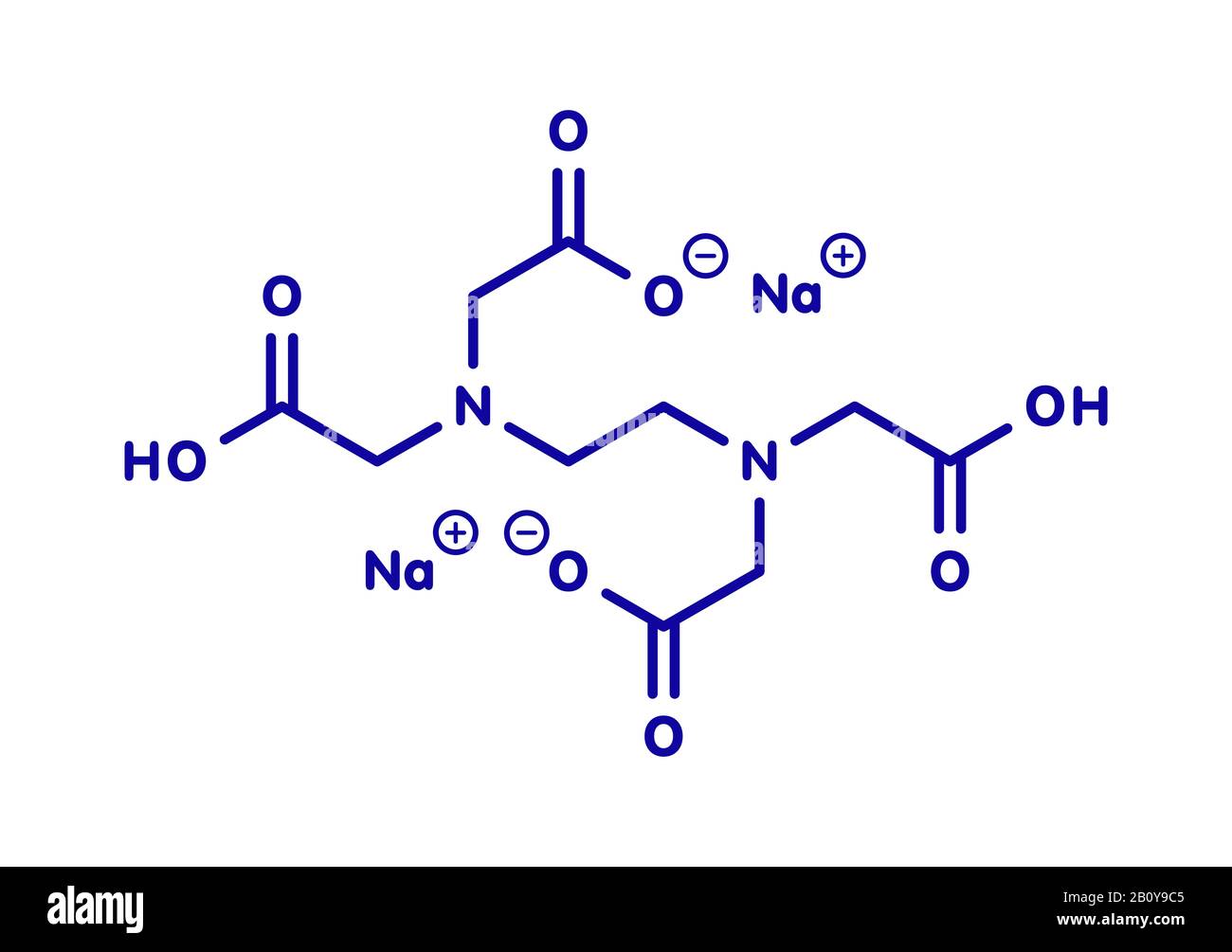
Edta High Resolution Stock Photography And Images Alamy

Indian Edta Disodium Salt Grade Aquaculture Purity Technical Id
Disodium Edta Hazardous Agents Haz Map
Edta Motm
Manganese Disodium Edta Trihydrate 84 5 Ir1
Edta

What Is Edta Disodium Benefits Side Effects Dosage

Tnn Edta Disodium Edta 2na Calcium Disodium Edta Tnn

Solved The End Point Of A Titration Was Reached After 28 Chegg Com
Edta Calcium Disodium 62 33 9

What Is The Structural Formula For Edta Quora
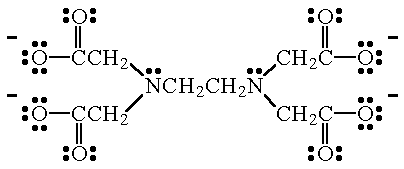
Coordination Compounds Help Page
What Is The Full Form Of Edta Quora

Experiment 5

Edetate Disodium Injection Empower Pharmacy

Mg Ii Edta Dojindo

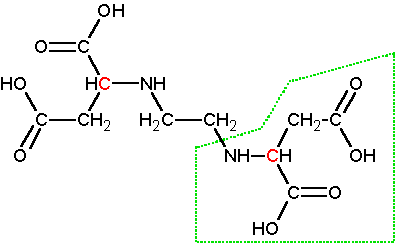
Structures Of Edta Disodium Salt Left And Metal Edta Complexes Right Download Scientific Diagram

Difference Between Disodium Edta And Tetrasodium Edta Compare The Difference Between Similar Terms

Edta Disodium Salt For Commerical Rs 290 Kilogram Meru Chem Private Limited Id

Ethylenediaminetetraacetic Acid An Overview Sciencedirect Topics

Calcium Disodium Edta Phar6157

Ethylenediaminetetraacetic Acid Wikipedia

Dual 0 02 Chlorhexidine Digluconate 0 1 Disodium Edta Loaded Thermosensitive Ocular Gel For Acanthamoeba Keratitis Treatment Sciencedirect

Edetate Disodium C10h14n2na2o8 Pubchem

Disodium Edta Water Softener And Chelating Agent Heritage Park Laundry Essentials

A Typical Chromatogram For The Mixture Of Disodium Edta Sorbic Acid Download Scientific Diagram
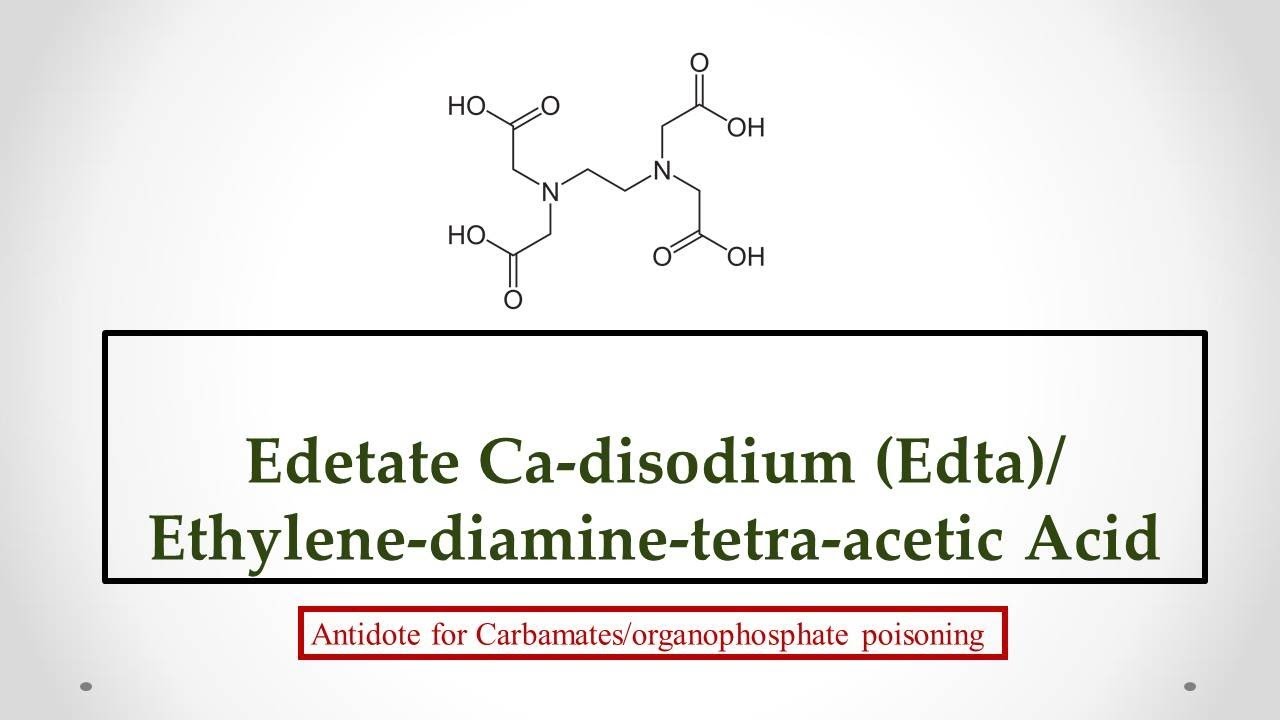
Edetate Ca Disodium Edta Uses Antidote Effects Mechanism Indications And Adr S Youtube

Edta Calcium Disodium Salt 62 33 9 Carbosynth Product

Edetate Calcium Disodium An Overview Sciencedirect Topics

Ethylenediaminetetraacetic Acid Wikipedia

Ingredient Spotlight Disodium Edta Futurederm
What Are The Structures Of Edta And A Metal Edta Complex Quora

High Quality Edetate Disodium Edta 2na Edta Disodium Salt Powder For Sale Buy Edta Disodium Salt Edetate Disodium Edta 2na Product On Alibaba Com
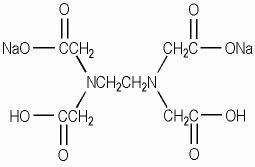
Disodium Edta Explained Products
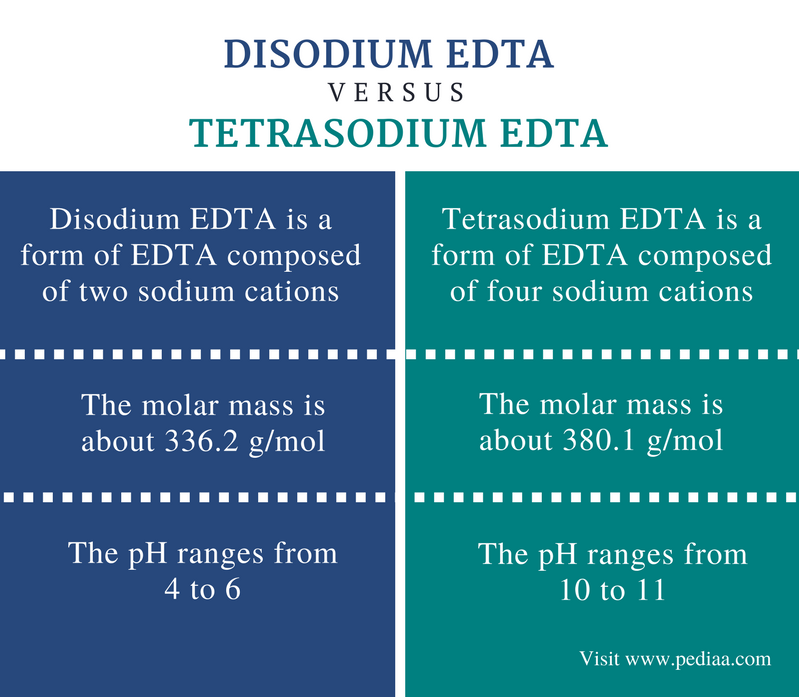
Difference Between Disodium Edta And Tetrasodium Edta Definition Structure Uses



